Home / Stories /
 by Catherine Arnold
by Catherine Arnold
October 28, 2021
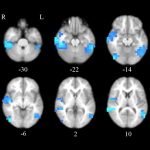
The genotypic and phenotypic complexity related to autism spectrum disorder (ASD) is extraordinary. Even mutations in single genes strongly associated with ASD, such as CHD8, generate substantial variability in outcomes. But if individuals experience the same mutation, why do their symptoms vary? To find out, a University of Washington team led by Rachel Earl, PhD, is learning more about affected individuals and their families.
“We know autism and other developmental disorders have a strong genetic component. By looking at parents and siblings of a child, adolescent, or adult affected with a rare gene mutation, we can start to understand more about the affected individual’s presentation,” says Earl, an acting assistant professor of psychiatry and behavioral sciences and a research affiliate at the Center on Human Development and Disability (CHDD). Earl also directs the Behavioral Evaluation Center at CHDD’s Eunice Kennedy Shriver Intellectual and Developmental Disabilities Research Center (IDDRC), where she oversees support for behavioral phenotyping for studies conducted by faculty investigators engaged in research on neurodevelopmental disorders.
Earl’s research on CHD8 is part of a NIMH-funded study (2019 to 2024) in which scientists are conducting genetic and clinical research with individuals and their biological parents and siblings, building on existing research with rare genetic mutations. Teams at the University of Washington, Pennsylvania-based Geisinger Medicine Institute, and Washington University in St. Louis are working to build better descriptions of the clinical patterns of individuals who share the same rare genetic disorder—while also identifying genetic factors in a person’s background that contribute to individual risk and resilience.
The Study
For the UW portions of the larger study, clinical assessments carried out at the CHDD are designed to gain an understanding of how individuals with the CHD8 mutation process information at different levels of analysis. In particular, the team aims to better understand the biological mechanisms of CHD8, how disruptions to the gene manifest clinically, and how to account for variability between individuals based on larger family genetic background. Genome sequencing is being carried out on blood, saliva, and other specimens from affected individuals and families.
The researchers located affected individuals by mining the electronic health record data at UW, Geisinger, and Washington University in St. Louis. Using the electronic health record sped up their process, noted Earl. Without it, they would have been forced to bring in a much larger group of individuals for testing.
Pandemic Changes and Increasing Equity
Normally, researchers in the three clinical centers contacted families and flew them into one of their respective locations to take part in 1-3 days of consultation and testing. When the COVID-19 pandemic hit, teams rethought their approach and now consult with families remotely, via videoconference sessions, phone calls, and online questionnaires.
This has given researchers the opportunity to reach a wider range of families, says Earl. “Families are often significantly impacted by their family member’s rare gene mutation. Whether families can take the time off to travel becomes an equity issue, but now we’re better able to meet families where they are.”
![]() Research Unique to University of Washington
Research Unique to University of Washington
As part of the NIMH-funded grant, UW researchers will acquire a knowledge of individuals with a number of genetic variants, with a particular focus on those affected by the CHD8 mutation. In 2014, Raphael Bernier, PhD, and others at CHDD published research in the journal Cell finding CHD8 to be a clear-cut autism associated gene change. The UW team established connections with more than 30 families who donate their time to help the researchers learn more about the genetic modification, says Earl.
In the current research, the team aims to understand what CHD8 does, what are its disruptions, and how to account for the ways its effects vary by family, Earl says. Comprehensive information is being collected, including the developmental, cognitive, and behavioral measures supported by the Behavioral Evaluation Center. The researchers anticipate that as the number of affected individuals participating in this research increase, a clearer phenotype and better insight into variability will emerge.
Accelerating Understanding to Improve Care
In graduate school, Earl became interested in genetics after working with families as part of her biology work. “I saw how varied individuals with autism can present, and I realized how varied our support needs to be.” The researchers hope to accelerate their understanding of these genes in order to provide better care, says Earl. “If we can quantify why genetic mutations have variable effects, maybe we can use that information to inform diagnosis and treatment, and find innovative ways to treat autism.”
Supporting Families
As a clinical psychologist and researcher, Earl works to highlight to researchers what is needed in order to make research in genetics accessible to families. “Genetics can feel like a foreign language for most people. In our clinical interactions, we want family members to feel they have more information about their child than when they came in.” Another benefit is that families in the research study often meet others who share their genetic experience. “The experience of living with their child’s challenges is often a difficult road,” says Earl. “Along with building our knowledge to improve care, seeing those connections fuels my passion for the work.”