Home / Stories /
 by Catherine Arnold
by Catherine Arnold
February 3, 2021
Down syndrome affects more than 6,000 babies born each year in the United States, including 1 in 700 newborns and even more pregnancies. From a scientific perspective, much has happened since 1959 when Jérôme Lejeune discovered individuals with the syndrome have an extra copy of chromosome 21. The condition is also referred to as Trisomy 21; the word “trisomy” describes a situation in which a person has three, instead of two, copies of a chromosome. Although this disorder has been deeply characterized since the 1950s, new and scalable technologies provide an opportunity for an even deeper dive. We are set to add new and unique knowledge to understand the earliest phases of development of Down syndrome at the University of Washington by building a Down syndrome atlas to visualize prenatal development at single cell resolution. The team of investigators consists of Kimberly Aldinger, Ph.D., senior scientist at Seattle Children’s Research Institute, Ian Glass, M.D., professor of pediatrics and medicine, and Dan Doherty, M.D., Ph.D., professor of pediatrics. Dr. Doherty directs the CHDD Genetics Core and he and Dr. Glass are both CHDD research affiliates.
Helping individuals with Down syndrome live fuller lives as they live longer is part of the research goal. Based on advances in research and clinical care, the average life span for a person with Down syndrome has been extended from 25 years in 1983 to 60 years. “It’s imperative for the research community to put more effort into developing therapeutic strategies to improve the lives of people with developmental challenges who are living longer than ever,” says Aldinger. The atlas is another step toward that goal.
Using methods the researchers and others previously employed to create atlases of fetal development in the labs of Jay Shendure, M.D., Ph.D., professor of genome sciences and Cole Trapnell, Ph.D, associate professor of genome sciences (see two recent 2020 publications in Science), the CHDD team is analyzing fetal tissue harboring trisomy-21 from the University of Washington’s Birth Defects Research Lab (BDRL) in collaboration with Shendure and his group. “Doing this work at early stages of development will allow us to identify differences in cell fate and gene expression that precede the medical and neurodevelopmental issues experienced by patients,” says Glass. “Even though we know development is not typical in Down syndrome, we really don’t know how that’s happening.” By examining early neural development, the researchers also hope to gain a better understanding of intellectual disability and early onset Alzheimer’s disease experienced by people with Down syndrome.
How The Down Syndrome Atlas Research Started
With the intention of learning more, in June 2018, the NIH launched the INCLUDE (Investigation of Co-occurring conditions across the Lifespan to Understand Down syndromE) project to fund new and continuing research on the condition. When Aldinger saw the NIH’s request for applications, she and team members looked through the logs at the BDRL, an NIH-funded source of donated fetal tissue important to medical research since 1959. Realizing they had access to quite a number of tissues expected to contain trisomy 21 from different time periods in fetal development, Aldinger led an effort to secure a grant through the INCLUDE program. “We realized we were in a strong position to establish a Down syndrome atlas using the BDRL tissues and the single-cell analysis approaches developed in the Shendure lab in UW Genome Sciences,” she said.
Technically Advanced Processing
The Shendure and Trapnell teams at UW Genome Sciences are well known for developing cutting edge genomic tools and analysis methods, including new assays and analysis software for single cell genomics. Through the UW Brotman Baty Institute, these “single cells by combinatorial indexing” approaches (or SCI) are available in a centralized lab to researchers who may not , otherwise have sufficient resources and knowledge to implement the methods. SCI approaches were first applied to cells, worms, and mice, which laid the groundwork required to initiate the Down Syndrome Atlas.
Examining Prenatal Development
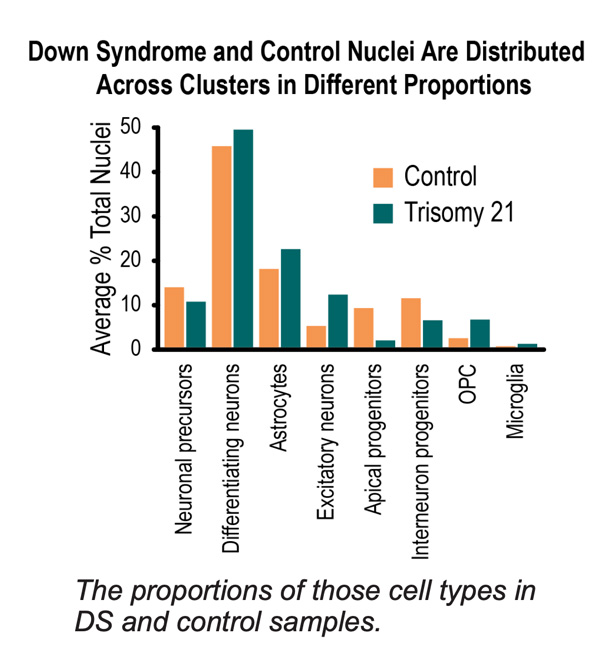
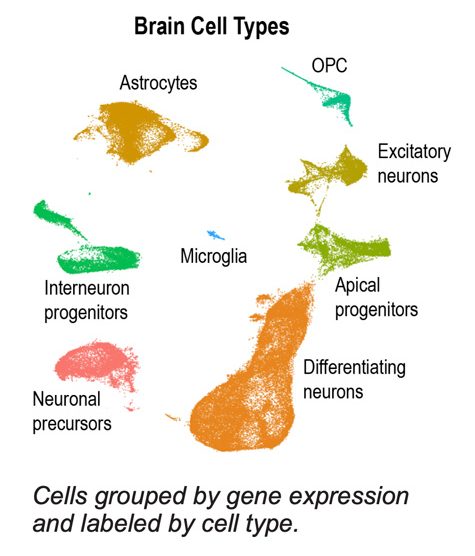 Currently in the analysis stage, the researchers have seen mainly subtle, not major, differences between Down syndrome cells and control cells, says Aldinger. Aware that the nervous system of individuals with Down syndrome develop and age differently (as shown by historical work and previous imaging studies) and that complications leading to birth defects can occur in the intestine, heart, and other organs, more often in Down syndrome, the team’s goal is to learn from cell development how those changes begin. With respect to brain development, they are examining if neurons are produced in the wrong place or at the wrong time in some cases or not enough neurons, or if some neurons may die or fail to connect correctly. While a body of evidence has demonstrated Down syndrome fetal developments with slower growth and smaller brains, the team’s analysis will allow them to learn more about how that happens. Mouse study findings have been limited for this area of brain development.
Currently in the analysis stage, the researchers have seen mainly subtle, not major, differences between Down syndrome cells and control cells, says Aldinger. Aware that the nervous system of individuals with Down syndrome develop and age differently (as shown by historical work and previous imaging studies) and that complications leading to birth defects can occur in the intestine, heart, and other organs, more often in Down syndrome, the team’s goal is to learn from cell development how those changes begin. With respect to brain development, they are examining if neurons are produced in the wrong place or at the wrong time in some cases or not enough neurons, or if some neurons may die or fail to connect correctly. While a body of evidence has demonstrated Down syndrome fetal developments with slower growth and smaller brains, the team’s analysis will allow them to learn more about how that happens. Mouse study findings have been limited for this area of brain development.
In addition, knowing that many individuals with Down syndrome have congenital heart abnormalities, the researchers are processing samples with and without prior evidence of congenital heart disorders. “By assaying different cell types, we will get a picture of which cell types are the most vulnerable,” says Aldinger.
Links to Adult Disease
Estimates indicate that 50 percent or more individuals with Down syndrome develop dementia due to Alzheimer’s as they age. Their extra chromosome carries a gene that produces amyloid precursor protein (APP), known as the Alzheimer’s protein. Too much of that protein leads to a buildup of clumps called beta-amyloid plaques in the brain. This process occurs in individuals with trisomy-21 at a much younger age, often in their forties, according to the NIH. Learning more about Down syndrome cellular development may yield insights on this disease process that could be applicable to the general aging population. For instance, if you eliminate APP from mice, they die. “There’s a requirement for APP in normal development even in mice,” says Aldinger. “In Down syndrome you may have too much of the gene that makes APP, and the effects of too much APP protein early in development are not clear yet.”
 In terms of cancer, vulnerabilities vary. Children with Down syndrome have a significantly increased risk of developing leukemia, with a form of pre-leukemia affecting up to 30 percent of newborns with trisomy-21—later regressing in most cases but developing into forms of leukemia for one quarter of that 30 percent. That said, individuals with Down syndrome have a lower rate of solid tumors. “Cancer involves re-activating development at an abnormal place and time—and Down syndrome has a propensity to develop one type of cancer but not another,” says Aldinger. “For me, that’s one of the scientifically fascinating aspects, the possibility that we can learn more about why one form of cancer takes place but another doesn’t.” Cell research will allow the team to look at transitions between diseases. “What we learn will help aging adults with Alzheimer’s and other diseases” says Glass.
In terms of cancer, vulnerabilities vary. Children with Down syndrome have a significantly increased risk of developing leukemia, with a form of pre-leukemia affecting up to 30 percent of newborns with trisomy-21—later regressing in most cases but developing into forms of leukemia for one quarter of that 30 percent. That said, individuals with Down syndrome have a lower rate of solid tumors. “Cancer involves re-activating development at an abnormal place and time—and Down syndrome has a propensity to develop one type of cancer but not another,” says Aldinger. “For me, that’s one of the scientifically fascinating aspects, the possibility that we can learn more about why one form of cancer takes place but another doesn’t.” Cell research will allow the team to look at transitions between diseases. “What we learn will help aging adults with Alzheimer’s and other diseases” says Glass.
Creating a Resource
Now that data generation is almost complete, the team expects to develop an atlas the research community can access on the website of the Brotman Baty Institute, says Glass. It’s time to get to the beginning of things in Down syndrome, notes Aldinger. “Although the cause of Down syndrome has been known for over 50 years, we haven’t had the tools until now to evaluate what’s happening and learn how to intervene. Now that we have those techniques, we’re excited and pleased to create a resource for other scientists to build on.”
