Home / Stories /
Sequencing Genes in a Complex Disorder: Decoding the Genetics of Epilepsy
 by Stacey Aggarwal
by Stacey Aggarwal
February 26, 2020
Developmental and epileptic encephalopathies (DEE) are a severe type of epilepsy characterized by multiple unprovoked seizures that can begin from the time of in utero development to a few years after birth. Repeated, severe seizure activity can lead to cognitive delays and related problems if not treated. However, many cases of DEE are not responsive to available therapies. Although seizure activity is generally caused by excess excitatory signals in the brain, exact causes of DEE remain poorly understood.
Recently, research has turned to genetics to better understand the etiology of epilepsy when brain malformations are not evident or when injury has not occurred. Heather Mefford, MD, PhD, professor of pediatrics and CHDD research affiliate, has been a leader in discovering how genetic changes influence this disorder. In describing the importance of genetic research in epilepsy, Mefford says, “Neurologists have more than 40 medications at their disposal, but a lot of the time they don’t work for these patients. Now, I think we’re in an era where we’re starting to use what we know about the biology or the molecular mechanism of a disorder to think more strategically about treatments – precision medicine. Understanding the genetics of these epilepsies will help us start to think about and develop more targeted medications that might be more helpful for these patients who really need better treatment.”
DNA Sequencing and Beyond
In her research program, Mefford is investigating the genetics of approximately 1000 individuals with developmental and epileptic encephalopathies based on either her work with the Epilepsy Genetics Clinic and the Neurology team at Seattle Children’s Hospital or through a longstanding collaboration with Dr. Ingrid Scheffer, a pediatric neurologist at the University of Melbourne in Australia. Mefford uses a variety of approaches to find genetic modifications in individuals with epilepsy that aren’t present in the genes of either of their parents.
One technique Mefford has used is exome sequencing. The exome describes the portion of the genome that contains exons – genetic regions known to encode functional molecules. With this technique, the entire exome is sequenced in affected individuals and compared to that of their parents to identify de novo genetic modifications. Similarly, Mefford and her team also look for epilepsy-related genetic modifications using targeted next-generation sequencing. This method sequences only a targeted panel of genes, rather than the entire exome, to optimize time and resources. The combination of these techniques allows Mefford to first screen for important genes, then selectively analyze those genes in a larger group of individuals.
Mefford is also using even more robust methods in this project to get a better picture of genetic – and even epigenetic – changes. Identifying genetic modifications outside of exons can be equally informative. “The exome, the gene-encoding part of our genome, is really only about 1% of our DNA,” Mefford points out. On discussing the utility of whole genome sequencing for her research, she says, “We have patients where we’ve sequenced the whole exome and haven’t identified a genetic cause for their epilepsy. So, in some of those cases, we’re going to try whole genome sequencing in both the patient and their parents.” Whole genome sequencing will not only help to identify genetic causes of epilepsy, but also inform researchers about important functions of lesser-understood regions of DNA. Finally, Mefford also plans to use high-throughput methods to find methylation changes to the DNA, an important epigenetic modification that can influence gene expression.
Gene Changes in a Few Can Paint a Broader Picture for Many
In practice, Mefford often starts by sequencing the whole exome or genome of a subset of individuals. Then, a deep comparative analysis of these sequences reveals suspect genes that are mutated or modified in epilepsy. She uses this information to create a smaller, targeted panel of potential epilepsy genes. This limited library of suspect genes is then used to screen the entire cohort. Mefford expands on the utility of this approach, “We do the most comprehensive test in a few individuals, and then we move to the larger cohort to do more selective, targeted testing in the rest.” This approach gives her a comprehensive yet efficient view of genes that are modified in epilepsy. Finally, after collecting this genetic information from the entire cohort, the results undergo stringent comparative analysis to highlight genes that may be pathologically altered in epilepsy.
Mefford describes her approach to discovering new, potentially pathogenic genes as follows: “We look at how many patients have changes in the same genes, how similar their clinical phenotype is, and how statistically likely it is that we would see this many new changes in the number of patients that we test for a given gene. It’s really a combination of clinical information and statistics.” When interesting genetic modifications are found, Mefford collaborates with other researchers to explore how these modifications influence epilepsy in model organisms.
Genes identified by Mefford’s research so far have been diverse in function. These include the chromatin-modifying gene CHD2, a GTPase known to be involved in synapse development SYNGAP1, and the ligand-gated chloride channel of the inhibitory GABA receptor GABRA1. She points out that, while some genetic modifications are found in families of genes that are known to be involved in epilepsy (such as ion channels and proteins involved in synaptic transmission), other broader processes have been implicated as well. In particular, Mefford’s research has also uncovered modification of genes involved in transcriptional regulation such as chromatin remodelers and transcription factors. She summarizes the takeaway from her findings so far, “These genes sort into several different bins – we’re finding that there are many different types of genes that, when modified, can lead to epilepsy.”
Moving Forward with Epilepsy
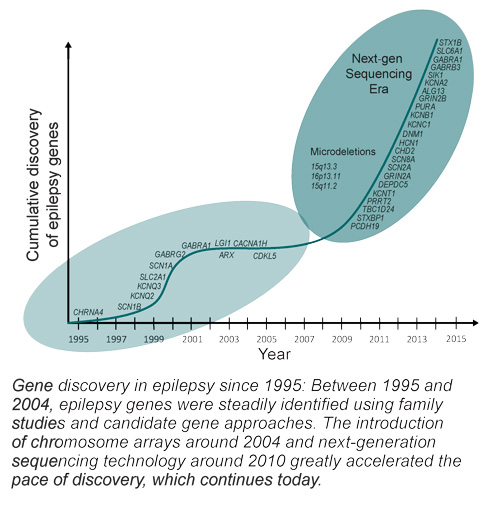 Mefford’s research has made large strides in understanding the genetic basis of epilepsy with next-generation genetic sequencing and analysis of copy number variants. While continuing these sequencing studies, she also hopes to add to these findings by expanding her genetic analyses even deeper. Using whole genome and exome sequencing, she hopes to get a clearer picture of the entire genome of individuals with DEE. Additionally, she is also eager to get a glimpse of the epigenetics of epilepsy by looking at DNA methylation. Given her earlier findings implicating the chromatin remodeler CHD2 in epilepsy, chromatin organization and epigenetics may be a significant component in the development of this disorder.
Mefford’s research has made large strides in understanding the genetic basis of epilepsy with next-generation genetic sequencing and analysis of copy number variants. While continuing these sequencing studies, she also hopes to add to these findings by expanding her genetic analyses even deeper. Using whole genome and exome sequencing, she hopes to get a clearer picture of the entire genome of individuals with DEE. Additionally, she is also eager to get a glimpse of the epigenetics of epilepsy by looking at DNA methylation. Given her earlier findings implicating the chromatin remodeler CHD2 in epilepsy, chromatin organization and epigenetics may be a significant component in the development of this disorder.
Highlighting the importance of studying the genetics of DEE, Mefford notes: “We study the most severe end of the spectrum – because it’s severe and very early onset; and without a brain malformation, birth trauma, or something else that might explain the seizures, we think that most cases will be due to a genetic cause.” She hopes that her research will help provide clarity to individuals and their families dealing with DEE. Furthermore, this intensive undertaking will inform future development of therapies for epilepsies that are resistant to currently available medications. Mefford believes that better understanding of the molecular mechanisms of epilepsy will help move research towards new and better treatment strategies.


